Clo3- Lewis Structure Molecular Geometry
ClO3- Lewis Structure, Molecular Geometry, Hybridization & Shape
The chemical formula ClO3 – represents Chlorate ion. Chlorine can reach oxidation states of +1, +3, +5 and +7. In this case, as seen in the figure, Chlorates be in a +five oxidation land.
With an abundance of oxidizing elements, the Chlorate ion and its salts brand for powerful oxidizing compounds. Potassium Chlorate (KCLOthree) is a ordinarily used oxidizing agent institute in diverse objects from disinfectants to pyrotechnics & explosives.
The compound has the following properties:
| Proper noun of the molecule | Chlorate (ClOthree –) |
| No. of valence electrons | seven+ (vi×iii)+1 = 26 valence electrons |
| Hybridization | sp3 |
| Bond Angles | 109.5° |
| Molecular Geometry of ClOthree – | Trigonal Pyramidal |
ClO3 – Valence Electrons
A Chlorate ion consists of one chlorine atom and iii oxygen atoms. There also exists a negative ionic charge of -1 in the ion.
Being in group 7 of the periodic table, Chlorine has seven valence electrons with a valency of -1. Hence the famed Cl– ion. Chlorine'south electronic configuration is given past [Ne]3s23p5. The possibility of electrons in its d shell makes it hypervalent.
Therefore, the single Chlorine atom contributes 7 x 1 = 7 electrons.
Being in group 6 of the periodic table, Oxygen has 6 valence electrons and has a valency of -2. Oxygen'southward electronic configuration is 1sii2sii2p4.
Therefore, given 3 oxygen atoms contribute 6 x iii =18 electrons.
The anionic accuse of -1 and contributes 1 valence electron.
Therefore, the total valence electrons in the chlorate ion is 7 + eighteen +one = 26
Lewis Structure of ClOthree –
The Lewis dot structures of an element represent a schematic system of its constituent molecules, atoms and their electron bonds.
The total number of electron pairs in a chlorate ion is given by:
26 valence electrons ÷ 2 = xiii pairs.
The Chlorine atom takes up a cardinal position in the structure due to its ability to share a college number of electrons. Therefore, information technology becomes a usher for more electron bonds than Oxygen hence taking up the center phase as shown in the figure.
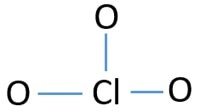
iii electron pairs are shown in the structure. The remaining pairs can be bundled according to the octet rule. The electrons are placed starting from the outside in. The three oxygen atoms accept in iii pairs each and the final pair is marked past Chlorine as shown in the figure.

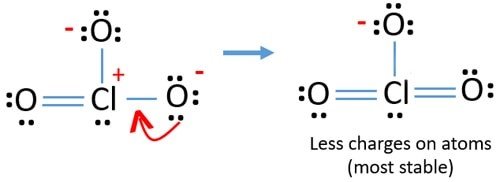
To decide the most stable Chlorate (ClOiii –) structure, we use the concept of formal charges. Formal charges for an element/structure help determine its nearly stable Lewis Structure land. Information technology is adamant such that the elemental charge on each cantlet is closest to zero.
FC = Valence Electrons – Non-bonding electrons – (Bonding electrons ÷ ii)
In this case,
| Element | 5 | N | B/two | FC |
| Cl | 7 | ii | 6/2 | +2 |
| O | vi | 6 | 2/ii | -1 |
Charges on these atoms every bit shown in the figure make it unstable. The higher the charges, the more unstable information technology is.
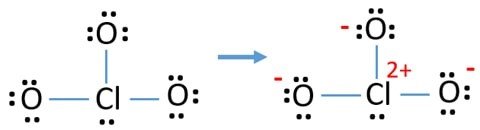
To combat this, we can transfer the lone pairs in the oxygen atoms to form bonds with Chlorine in the middle. This gives Chlorine more than than 8 electrons in the center. This is feasible due to Chlorine's empty 3d orbits, which make it an exception to the octet dominion.
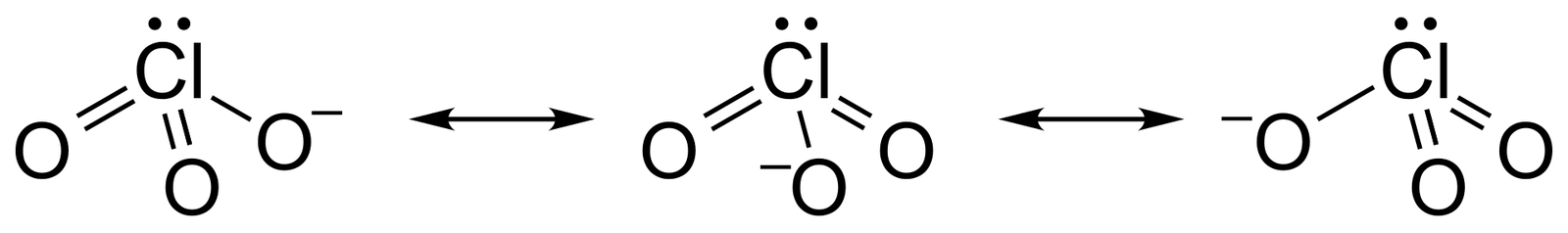
Chlorate ions exist in multiple resonant states due to the shifting of bonds as shown in the figure.
ClOthree – Hybridization
The key atom, Chlorine (Cl), forms covalent bonds of double and single nature with the surrounding atoms of Oxygen. To calculate ClO3 – hybridization state, we need to determine the number of lone pairs and the number of sigma bonds nowadays in the ion.
Since Chlorine is in the third row in the periodic table, it is capable of expanding its orbitals in an excited state. Its electronic configuration is given by [Ne] 3s2 3pv.
Therefore, the two lone pairs fill in the 3s orbital and the three sigma bonds present make full-up the 3p orbital. At present, nosotros insert the ii pi bonds into the beginning two 3d orbital spaces.
The rest of the electrons from the sigma bonds are filled into the 3p orbital.
Therefore, the hybridization of ClO3 – is sp3.
ClOthree – Bond Angles
Due to the repulsion of electrons on the surrounding oxygen atoms and the solitary pair on Chlorine, the atoms become pushed apart and form bond angels of 109.5°.
ClO3 – Molecular Geometry and Shape
Chlorine forms the fundamental atom in the ClOthree – ion with oxygen atoms surrounding it. Co-ordinate to the VSEPR theory (Valence Shell Electron Pair Repulsion), the lonely pair on Chlorine and the electron clouds on the surrounding oxygen atoms repel each other.
Since nosotros know that the steric number of ClO3 – is 4 (1 solitary pair and three bonded pairs), we tin decide that the Molecular geometry of ClO3 – is Trigonal Pyramidal.
From the Lewis construction above, we know that its electronic shape is tetrahedral.
Concluding REMARKS
Let'south apace summarize the salient features of ClO3 –
- ClOiii – consists of one Chlorine cantlet and iii Oxygen atoms
- In its most stable state, Chlorine forms three covalent bonds with the surrounding Chlorine atoms making for three bonded pairs in the center with a lone pair of Chlorine.
- ClO3 – has an sp3 hybridization state.
- ClO3 – has a trigonal pyramidal structure with bond angles of 109.five°.
Clo3- Lewis Structure Molecular Geometry,
Source: https://geometryofmolecules.com/clo3-lewis-structure-molecular-geometry-hybridization-shape/
Posted by: rayburntrom1949.blogspot.com


0 Response to "Clo3- Lewis Structure Molecular Geometry"
Post a Comment